QUESTION
Assignment 1
Define the lowest observed adverse effect level (LOAEL) and the no observed adverse effect level (NOAEL), and describe how they relate to the point of departure (POD). Describe how each of these are used to derive an OEL.
Your answer must be a minimum of 200 words in length. 2-3 refernces with intext in APA style
Assignment 2
Discuss the common elements used to derive occupational exposure limits (OELs). Provide your opinion as to which of the elements is the most important.
Your answer must be a minimum of 200 words in length. 2-3 refernces with intext in APA style
Assignment 3
hat are some chemical and/or biological hazards in the workplace that you might have come in contact with there? If you had been or were currently an industrial hygienist or safety officer at the organization in question, how would go about ensuring that the health effects of those hazards were properly mitigated?
Your journal entry must be at least 200 words. No references or citations are necessary.
Assignmnet 4
Message Body:
Write a minimum of one page for each hazard you choose (a minimum of three pages total), which summarizes the following information. One substance must be a gas/vapor hazard, one must be an aerosol hazard, and one must be a biological hazard.:
- Explain whether the substance is a chemical or biological hazard, and explain how you determined that.
- Explain the key chemical properties (vapor pressure, vapor density,molecular weight, relative size) as applicable, and describe how theseproperties affect the different routes of exposure. Based on the chemicalproperties, how would you identify which exposure route is the most important?
- Analyze how the substance could enter the body through the dermal route,and discuss why the dermal route would or would not be important.
- Describe the region of the respiratory system where deposition would be expected (only for the aerosol hazard).
The title page and reference page do not count toward meeting the required page count.
800 words excluding refernces and title page word count, 5-6 references with intext…
ANSWER
ASSIGNMENT 1
Lowest Observed Adverse Effect Level (LOAEL) and No Observed Adverse Effect Level (NOAEL) refer to the actual doses used in human experimental or clinical studies for the analysis of effectiveness of doses on the animal. Both of these are represented through a dose-response curve.
LOAEL is the lowest dose at which there was an observed toxic or adverse effect on the subject; whereas NOAEL is the highest dose at which there was not an observed toxic or adverse effect on the subject. Point of Departure (POD) is the point on a toxicological dose response curve established from an experimental data corresponding to a no effect or estimated low effect level.
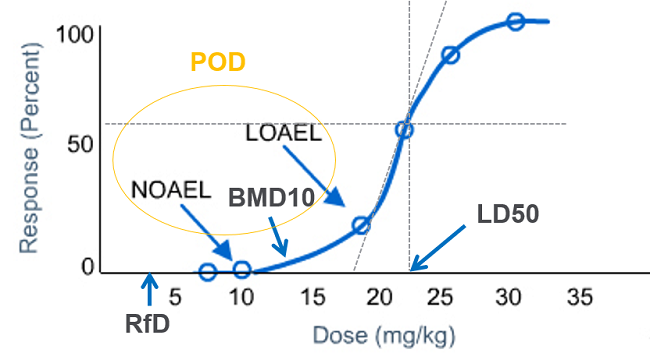
NOAEL is used as POD to calculate RfD (Reference Dose) in case of toxicological effects where extensive testing has failed to identify a threshold and there is not enough data to develop a statistical model. When NOAEL is not available, then LOAEL is used as POD for RfD calculation.
Both NOAEL and LOAEL are used to calculate OEL by using human body weight, breathing rate of workers, and safety factor. The safety factor here can be a multiplicative effect of Composite Uncertainty Factor, Toxicokinetic Adjustment, and Modifying Factor.
OEL = [(NOAEL) * (Human Body Weight)/ (Safety Factor * Human Breathing Rate)]
When NOAEL is not available, then LOAEL is used to calculate OEL.
References
1. Robert H. Ku, Feb 2000, An Overview of Setting Occupational Exposure Limits (OELs) for Pharmaceuticals, SafeBridge Chemical Health & Safety
2. D. A. Dankovic, 2015 Nov 25, The Scientific Basis of Uncertainty Factors Used in Setting Occupational Exposure Limits, Journal of Occupational and Environmental Hygiene
ASSIGNMENT 2
The common elements which are used to derive Occupational Exposure Limits (OELs) are POD (Point of Departure), Human Body Weight, Safety Factor, and Human Breathing Rate.
POD is the point on a toxicological dose response curve which is established from experimental data corresponding to a no effect or estimated low effect level. It can be calculated using (a) The Benchmark Dose Approach, (b) T25 Approach, (c) TD50 Approach, or (d) NOAEL/LOAEL Approach. BD Approach follows dose response models developed from all available data on dose response curve, whereas T25 is based on one data point from dose-response curve. TD50 considers the linearity of dose and hazard, whereas NOAEL/LOAEL are based on exposure level in case of statistically significant adverse effects. This is the most important factor as it decides the extrapolation of Reference Dose and the effectiveness of experimental concentration of dose.
Human Body Weight is typically assumed to be the average weight of an adult male.
Safety Factor includes a no of other factors such as – (i) Composite Uncertainty Factor (which is a multiplicative factor of five uncertainties i.e. Interspecies variability in response due to extrapolation of animal studies to humans, No effect level uncertainty estimation, Response variability in human beings, Extrapolation uncertainty due to full life-time studies exposure based on shorter duration studies, and Other insufficiencies in the overall health effects database), (ii) Modifying Factor (which handles exposure scenarios due to interplay between the above five uncertainty factors), and (iii) Toxicokinetic Adjustment (which handles additional toxicokinetic adjustments such as route-specific bioavailability or route-to-route extrapolation factor).
Human Breathing Rate is the breathing rate of workers during their work duration.
Reference
1. M. Deveau, 2015 Nov 25, The Global Landscape of Occupational Exposure Limits—Implementation of Harmonization Principles to Guide Limit Selection, Journal of Occupational and Environmental Hygiene
2. Gunnar Johanson, 2001 Sep 28, Occupational exposure limits – approaches and criteria, National Institute for Working Life Sweden
ASSIGNMENT 3
Airborne chemical hazards exist as concentrations of vapours, gases, mists, liquids, or fumes. Some of it are toxic through inhalation, some are irritable for skin in contact, while some are corrosive to living tissue. Biological hazards are caused because of exposure to virus, fungi, bacteria, or parasites that can cause chronic and acute infections by entering the body either directly through cuts, bites, or touches, or through breaks in the skin.
Some of the common chemical hazards are pesticides, paint, acids, heavy metals (Lead, Mercury, Aluminium, etc), and cleaning products such as toilet cleaners, chlorine bleach, disinfectants. For avoiding these hazards, workers should be instructed to keep harmful chemicals in sealed containers along with proper labelling and to open it under specific conditions only. The workers should wear hand gloves and masks while dealing with any chemical. The company should classify chemicals and conduct proper risk assessment measures for each chemical with a regular review system.
Some of the biological hazards that can happen are Pandemic Influenza, Rabies, Needlestick and sharps injuries, Hepatitis, Common cold, Bed Bugs, etc. There are a few things to keep in mind for workers to avoid biological hazards such as maintaining proper personal hygiene and attention to minor cuts or scratches on the hand while working, washing hands properly after work, using adequate infectious waste disposal systems, wearing proper personal protective equipment such as helmets, gloves and respirators while working.
1. John Shimwell, 2001 Apr 01, Occupational Health – A manual for primary health care workers, WHO
2. Walter Ingles, 2019 Jan 16, Chemical hazards in the workplace, Storemasta
ASSIGNMENT 4
We have been asked to choose three hazards out of which one must be gas/vapor hazard, one must be aerosol hazard, and one must be a biological hazard.
The chosen gas/vapor hazard is –
Ammonia
Ammonia can be considered as a chemical hazard. The chemical formula of Ammonia is NH3. The pure form of this chemical, i.e. Anhydrous Ammonia, is a highly hazardous chemical with corrosive and flammable nature, and a potential reason for human health risk.
Vapor Pressure of Ammonia = 6658 mm of Hg
Vapor Density of Ammonia = 0.59 g/cm3
Molecular weight = 17 g/mol
Relative sizeionic = 175 pm
Different routes of Exposure –
Skin Contact – Upon direct contact with concentrate solution of Ammonia, skin can be burnt or irritated, resulting in a permanent scar. Liquefied gas can freeze the skin upon direct contact, which is commonly known as Frostbite. It may also cause a burning sensation and stiffness, resulting in a yellowish or waxy white colour of skin. In severe cases, tissue death, blistering, and infection can also develop.
Mucus Membranes – Ammonia irritate and burn eyes, with permanent blindness developing in severe cases. This route of exposure is very common as the vapor pressure and density allows its easy movement in air.
Inhalation – Ammonia has the potential to cause severe irritation of the nose and throat and it can also cause life-threatening accumulation of fluid in the lungs (called Pulmonary Edema). In severe cases, high concentration of Ammonia inhalation can also cause death. This is the most common route of exposure for Ammonia due to its relative size and vapor density.
Ammonia can enter the body through the dermal route and can cause chemical burns. For example, Industrial Cleaners contain around 25% concentrated Ammonia solutions and can cause serious corrosive injuries to the skin. Dilute aqueous solutions with less than 5% Ammonia concentration rarely cause serious burns but it can be moderately irritating. However, higher concentrations may result in pain, inflammation, necrosis, blisters, and deep penetrating burns, especially on moist skin areas.
The chosen aerosol hazard is –
Crystalline Silica Dust (Quartz, Cristobalite)
With the chemical formula as SiO2, this substance could be considered as a chemical hazard. This chemical can cause damages to lungs, kidneys, and even cancer in severe cases. Both Quartz and Cristobalite are chemical polymorphs which are used in construction business. They both share the same chemical formula but different structure.
Vapor Pressure = Approximately 0 mm of Hg
Vapor Density = 2.33 g/cm3
Molecular weight = 60.083 g/mol
Relative sizeionic = 55 nanometres
Different routes of Exposure –
Inhalation – It is the primary route of exposure for Crystalline silica dust, with their nanoparticles having the potential threat of deep penetration into the lungs. Even a very small amount of fine respirable silica dust can cause serious health hazards for the employees. Different amount of respirations may have different impacts such as Chronic silicosis, Accelerated silicosis, and Acute silicosis are there based on the duration of respirable crystalline silica exposure to workers.
Water Drinking – It happens when someone is living near any construction site and drinking the available ground water, which is exposed to silica dust. Regular consumption of silica can cause long-term illness for the people, resulting in some serious diseases.
Skin Contact – This is not a major exposure route for silica dust. Hands and face can be irritated when it comes in contact with silica dust for a few minutes. But apart from irritation and mild burning in some cases, there is not enough hazardous impact on skin.
Crystalline silica dust cannot enter into the body through dermal contact directly. However, if hands have been exposed to silica dust for some time and then the person eats food using that hand, then it can enter into the body. Skin irritation also occurs in some of the cases of direct contact with silica dust. Overall, the dermal route is not that important for silica dust to harm a person, until and unless he/she is not maintaining personal hygiene and regularly washing hands and legs after work.
Crystalline silica dust nanoparticles embed themselves into the alveolar sacs in the lungs. These are the cells where Oxygen and Carbon dioxide are exchanged.
The chosen biological hazard is –
Bronchitis occurring due to Nitric Acid
Nitric Acid could be considered both a biological and chemical hazard. Bronchitis is one of the most common hazardous disease occurring due to exposure to Nitric acid. Bronchitis would be considered a biological hazard as it is a biological (viral) living organism.
Vapor Pressure of Nitric Acid = 48 mm Hg (at 20o C)
Vapor Density of Anhydrous Nitric Acid = 1.513 g/cm3
Molecular Weight = 63 g/mol
Relative Size = 40 to 100 nanometres
Different routes of Exposure –
Inhalation – It is a very common occupational hazard as the substance readily forms a vapor at room temperature. Exposure to nitric acid in the air could cause effects such as dry nose and throat, chest pain, cough, headache, shortness of breath, and difficulty in breathing.
Skin/Eye contact – Nitric acid is an extremely corrosive acid which if gets in contact with skin can result in a severe corrosive burn, with the intensity depending upon the concentration of acid. The vapor is very irritating to the eyes, throat, and corrosive to the teeth.
Ingestion – Nitric acid is harmful if ingested and it can result in burning and corrosion of the mouth, throat, and stomach. An oral dose of even 10ml is considered fatal for humans. Gastritis, oesophageal and gastric burns can also occur in some cases.
Dermal contact is very dangerous for this substance, as it can severe corrosive burns and irritation to the person. Nitric acid can come in contact in either solution form or powder form. Ulceration, Dermatitis, and yellow staining of skin might also develop in some cases of dermal contact.
References
1. National Center for Biotechnology Information, 2004 Sep 16, Compound Summary of Ammonia, PubChem Compound Database
2. Agency for Toxic Substances and Disease Registry, 2014 Oct 21, Medical Management Guidelines for Ammonia, US Division of Toxicology and Human Health Sciences
3. New Jersey Department of Health, 2016 Feb 04, Ammonia – Hazardous Substance Fact Sheet, US Department of Health and Human Services
4. William R. Effland, 2010 Apr, Paedogenic and Biogenic Siliceous features, Science Direct
5. Claire J. Horwell, 2012 Nov 19, The structure of volcanic cristobalite in relation to its toxicity; relevance for the variable crystalline silica hazard, US National Library of Medicines
6. F L M Ricciardolo, 2003 Feb, Multiple roles of nitric oxide in the airways, BMJ Journals
7. T. K. Jayalakshmi, 2009 Dec 24, Acute lung injury following exposure to nitric acid, NCBI
Looking for best Nursing Assignment Help. Whatsapp us at +16469488918 or chat with our chat representative showing on lower right corner or order from here. You can also take help from our Live Assignment helper for any exam or live assignment related assistance.